Life
Sciences
We provide timely and accurate drug development and commercialization services from our U.S. owned and operated CDMO/CRO network.
improving our health
We believe the therapies our customers develop are critical to improving lives and we are proud to be a part.
Pace® Life Sciences Hosts Grand Opening of its Center of Excellence for Aseptic Fill-Finish Services in Salem, NH
July 16 open house highlights expanded sterile filling contract manufacturing capabilities for small and large molecules
Pace® Life Sciences Announces Successful Pre-Approval Inspection from US FDA in Lebanon, NJ
Pace® Life Sciences announced today a successful Pre-Approval Inspection (PAI) from The Food & Drug Administration (FDA) highlighting company’s commitment to reliable and accurate data.
Future role of Pace® Life Sciences in supporting biopharmaceutical development and manufacturing coast-to-coast – Dean Bornilla, Vice President.
Pace® Life Sciences, a biopharmaceutical services provider, has experienced significant growth through recent acquisitions of analytical labs, substantially increasing their testing capacity and scientific expertise.
Pace® Life Sciences Awarded Bronze Medal from EcoVadis for Commitment to Sustainability
Pace® Life Sciences, is pleased to announce that it has received a Bronze Medal award from EcoVadis for its current sustainability initiatives. This recognition places Pace® in the top 35% of over 130,000 companies evaluated for their commitment to sustainability.
Addressing the Complexity of Modern Therapeutics – Dr. Frank Tagliaferri, Pace® Life Sciences
The modern therapeutic landscape is defined by an ever-growing portfolio of complex drug products – from biologics and gene therapies to mRNA-based vaccines and monoclonal antibodies.
Sterile-Fill Finish Manufacturing Expansion
The demand for fill-finish manufacturing continues to grow due to the rising number of biologics and gene therapy products being developed, predominantly for parenteral administration. The increasing introduction of more convenient and user-friendly routes of administration for these products has also significantly contributed to the demand for more manufacturing capacity.
Pace® Life Sciences recognizes the underlying market drivers influencing our clients’ advancements, which informs our latest investments to expand sterile filling contract manufacturing capabilities in Salem, NH. Explore the official debut of our Sterile Fill-Finish Center of Excellence. We look forward to helping advance your program to the next phase.
Explore our range of services
Wherever you are in your drug development journey, we can help.
THE RIGHT PARTNER FOR YOUR PROJECT
We have a nationwide network of state-of-the-art facilities, each with long-established histories of successful product development and commercialization and excellent audit outcomes from regulatory agency and client reviews.
FDA
REGISTERED
DEA
DEA Schedules I – V
GMP
COMPLIANT
ISO
17025 ACCREDITED
OUR PROMISE TO YOU
We honor our commitments so you can honor yours™. Our investment in state-of-the-art facilities and highly trained experts emphasizes our commitment to delivering positive customer experiences across all phases of pharmaceutical and biopharmaceutical development.
- RELIABLE DELIVERY
- COLLABORATIVE RELATIONSHIPS
- EXCEPTIONAL SERVICE
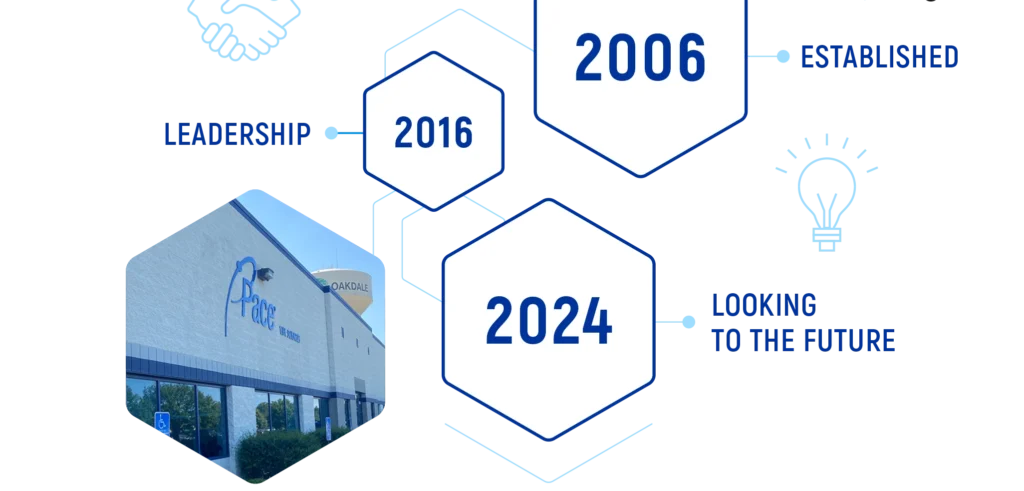
IT ALL STARTED IN 2006
Since 2006, Pace® Life Sciences has continued to prioritize strategic investments and domestic acquisitions to meet the changing needs of our customers. As the market changes, we are committed to making sure we are positioned as the best U.S. owned and operated end-to-end solution for your program.
Conferences

PRChem 2025
PRChem 2025 offers the main chemistry conference in the Caribbean and it will bring together participation from the private sector, government, industry, academia and other professionals.
July 30 – August 1, 2025 | San Juan, Puerto Rico
feature webinar

Overview of FDA’s Expedited Programs for Serious Condition
Recognize the importance of expedited review programs, such as FTD, BTD, and RMAT Designation, and their impact on reducing drug development time.
Now available on demand








