Agile Clinical Manufacturing of Injectable Biologics
Navigate the complexities of sterile injectable manufacturing with confidence. Our team has the expertise and equipment needed to ensure regulatory compliance and meet your clinical supply demands for injectable biologics. Together, we’ll overcome the common manufacturing challenges, keeping your program’s progress on track.
Whether your modality is a protein, peptide, vaccine, or mAbs, our team has the expertise, infrastructure, and resources to fulfill your clinical supply needs. With diverse and adaptable operations, we seamlessly fill your biotherapeutic into any standard vial or syringe with manual or automated fill-finish systems. Your product’s safety and sterility are of utmost importance to us – filled materials undergo release testing and examination by our QC and QA teams before shipment to the clinical site. Our onsite project management teams expedite testing and batch release to consolidate your support requirements. Trust our continuously expanding capabilities to meet your requirements through both your clinical and commercial campaigns.
Sterile-Fill Finish Manufacturing Expansion
The demand for fill-finish manufacturing continues to grow due to the rising number of biologics and gene therapy products being developed, predominantly for parenteral administration. The increasing introduction of more convenient and user-friendly routes of administration for these products has also significantly contributed to the demand for more manufacturing capacity.
Pace® Life Sciences recognizes the underlying market drivers influencing our clients’ advancements, which informs our latest investments to expand sterile filling contract manufacturing capabilities in Salem, NH. Explore the new Sterile Fill-Finish Center of Excellence making its official debut July 16, 2025.
Clinical Manufacturing
Navigate contamination risks during your pharmaceutical production process with our clinical manufacturing infrastructure. Our expert team and specialized equipment meet regulatory compliance, scalability, and quality assurance requirements. Our aseptic fill-finish manufacturing facility meets FDA, cGMP, and EU requirements for phase I and II clinical trial products. This facility is monitored, controlled, and recorded in real-time and serviced with our own Water for Injection (WFI) system. Our HVAC system uses 99.99% efficiency HEPA (High-Efficiency Particulate Air) filters. It is housed in an easily serviced mezzanine above the fill suite and below our roof, eliminating weather-related malfunctions.
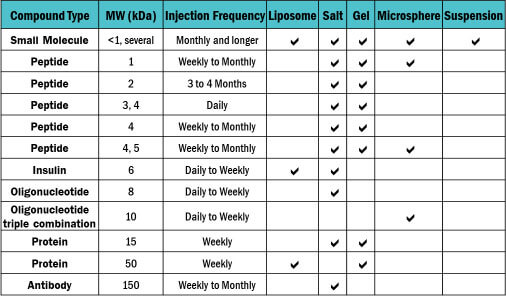
Choosing the right vehicle for your drug substance can be a complex and critical decision, impacting factors like formulation stability and bioavailability. With experience across multiple vehicle types, our team is here to help you select the most compatible vehicle for your drug substance that will provide for a successful manufacturing process.
During early-stage research, you may face limitations with the accessibility of clinical trial materials. Our ability to produce small batch sizes not only helps conserve your valuable biological product but also saves you time and money. We have a track record of achieving consistently high yields without compromising quality, even when working with limited starting materials.
Creating a comprehensive batch record is critical to meet regulatory requirements and ensure you have a successful clinical phase. Our batch record outlines and documents every step involved in manufacturing your product for clinical use, crucial for the Chemistry, Manufacturing and Control (CMC) section of an Investigational New Drug (IND) Application. We also execute smaller scale engineering runs to proactively address any potential challenges that may arise during full-scale production.
When it comes to clinical trial materials, cost-effective solutions are key to sustain your program. We provide you access to validated bottles and vials, offering substantial cost savings when they are suitable for your needs. Choose from our various vial sizes, including 2R, 4R, 6R, 10R, and 20R. We flexibly accommodate batch sizes up to 10 to 20 liters.
Integrated Laboratory Services
As your project approaches pivotal phases, ensure you have the comprehensive support needed to maintain progress. Our experts and operations offer the insight and capacity to provide a customized solution.